The Public Comment is now open through March 25, 2026 on the Draft 2027 CMS QRDA I Implementation Guide, Schematron, and Sample File for Hospital Quality Reporting
Top
Resources Subnav
The QDM is a conceptual information model that defines clinical patient data and concepts in a standardized format to enable electronic quality performance measurement. The model is the current structure for electronically representing quality measure concepts for stakeholders involved in electronic quality measure development and reporting. Electronic health record systems can use the QDM to consistently understand the information to access from clinical records to report the data required to meet measure criteria.
CMS established the Quality Data Implementation (QDI) User Group to ensure broad stakeholder collaboration in quality measurement standards development, maintenance, and harmonization with quality measure and Clinical Decision Support (CDS) standards to support the electronic clinical quality improvement (eCQI) landscape. Participants of this open stakeholder group use their expertise to provide feedback and influence electronic data modeling based on
- measurement needs
- real-world feasibility
- harmonization with Health Level Seven International® electronic clinical quality measure (eCQM) and CDS standards.
A broader focus of this group now allows for discussion around emerging quality measurement standards like Fast Healthcare Interoperability Resources® (FHIR®) and Quality Improvement (QI) Core in addition to the QDM data model.
You can review the QDI User Group Charter for more information about the group. To get involved or provide feedback, refer to the Connect tab.
Overview
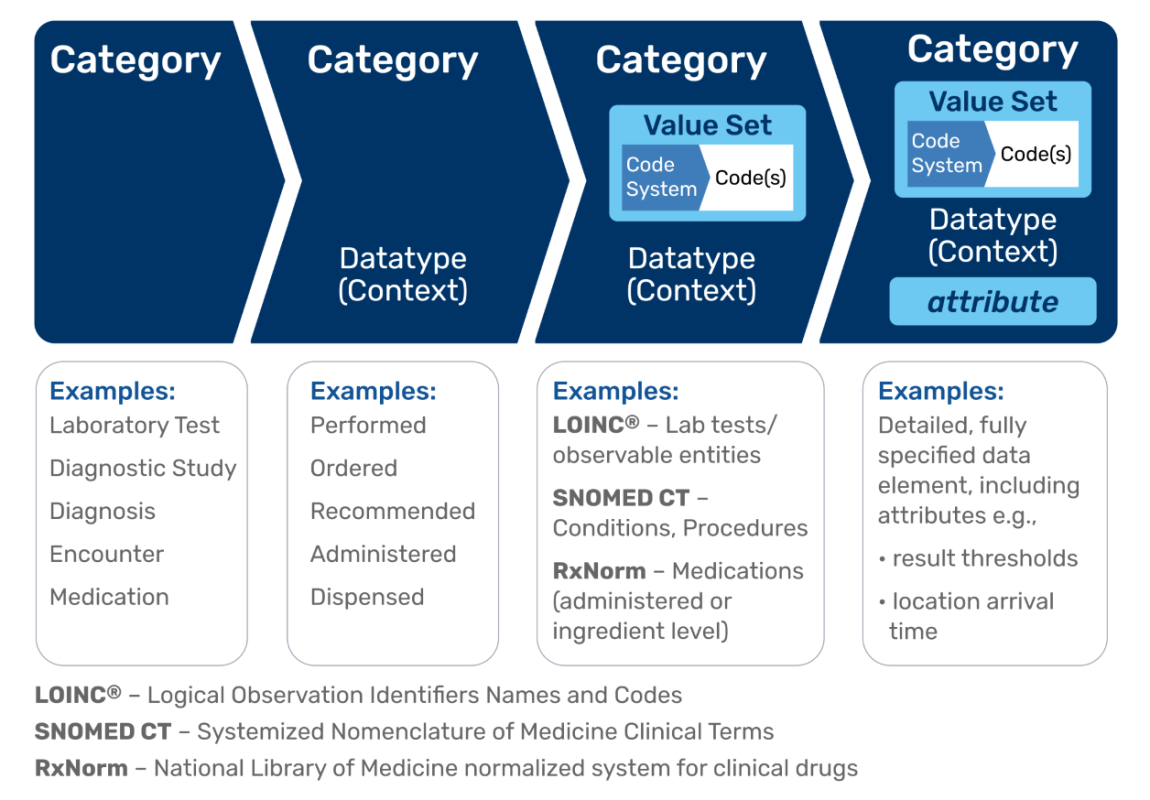
The QDM provides the language that defines the criteria for clinical quality measurement. The graphic below shows how QDM allows for the electronic definition of a clinical concept via its data elements and provides the vocabulary to relate these elements to each other. By relating attributes between data elements and using filtering functions, the QDM provides a method to construct complex clinical representations for eCQMs. The QDM provides the foundation for the conceptualization and specification phases in the eCQM Measure Lifecycle.
Category - Consists of a single clinical concept identified by a value set. A category is the highest level of definition for a QDM element. QDM 5.6 contains 22 categories. Some examples of categories are Medication, Procedure, Condition/Diagnosis/Problem, Communication, and Encounter.
Datatype - The context in which each category describes a part of the clinical care process. Examples of QDM datatypes include “Medication, Active” and “Medication, Administered” as applied to the QDM Medication category.
Attribute - Provides specific details about a QDM data element. QDM data elements have datatype-specific attributes.
- Datatype specific attributes - Datatype-specific attributes provide detail about a QDM data element based on its datatype. For example, “Medication, Dispensed,” “Medication, Order,” and “Medication, Administered” all contain information about dosage, supply, frequency, and route. “Medication, Dispensed” and “Medication Order” include the attribute refills, but “Medication, Administered” does not. Because these attributes pertain to specific datatypes, they are called datatype-specific attributes. Starting with QDM version 5.5 and continuing with subsequent versions, each QDM datatype includes an actor to allow reference to the individual or organization performing the activity. The QDM documentation provides definitions of all QDM attributes including the new actor attributes.
Entities - QDM 5.5 and subsequent versions introduced a new concept called QDM Entities. Entities are not QDM datatypes or attributes. Use entities to represent concepts to specify details about the actor (or performer) of any QDM datatype. An eCQM can use the entities to provide further information required for an individual or organization actor to meet the measure’s criteria. Entities include Patient, Care Partner, Practitioner, and Organization. QDM 5.6 provides examples of how to use these new QDM Entities.