The Public Comment is now open through March 25, 2026 on the Draft 2027 CMS QRDA I Implementation Guide, Schematron, and Sample File for Hospital Quality Reporting
Top
eCQMs Subnav
Electronic clinical quality measures (eCQMs) are measures specified in a standard electronic format using data electronically extracted from electronic health records (EHRs) and/or health information technology (IT) systems to assess the quality of health care provided. The Centers for Medicare & Medicaid Services (CMS) uses eCQMs in a variety of quality reporting and value-based purchasing programs.
There are several benefits of using eCQMs:
- eCQMs use clinical data enabling more accurate assessment of treatment outcomes by measured entities to assess the outcomes of treatment by measured entities.
- eCQMs use electronic standards, which help reduce the burden of manual abstraction and reporting for measured entities.
- eCQMs foster the goal of access to real-time data for point of care quality improvement and clinical decision support.
Measured entities use eCQMs to provide feedback on their care systems and to help them identify opportunities for clinical quality improvement. Measured entities report eCQMs to CMS, The Joint Commission, other federal health agencies, and commercial insurance payers in programs that track and/or reimburse measured entities based on quality reporting or quality performance.
Review eCQM Basics, eCQM 101 - Getting Started with eCQMs for Quality Reporting Programs, and visit the eCQM Annual Timeline and Educational Resources pages to learn more about eCQMs.
Explore CMS Measure Types
Find eCQMs
Report eCQMs
For CMS programs, hospitals and/or eligible clinicians must use the most current version of eCQMs (measure specifications) when reporting eCQMs to CMS.
- For hospital inpatient reporting guidance, visit QualityNet and the Quality Reporting Center for specific program reporting education.
- For hospital outpatient reporting guidance, visit QualityNet and the Quality Reporting Center for specific program reporting education.
- For clinician reporting guidance, visit the Quality Payment Program website and the Quality Payment Program Resource Library.
- For federal health agency programs using eCQMs, refer to specific program requirements.
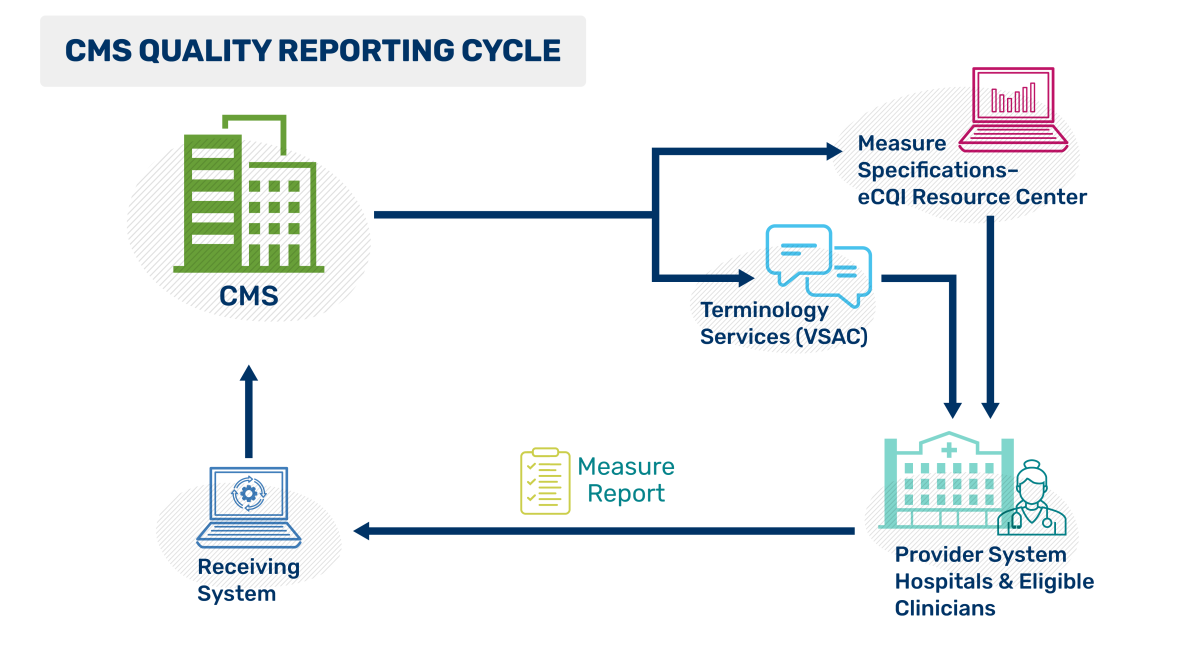
This CMS Quality Reporting Cycle figure depicts the flow of quality reporting as a continuous loop. CMS creates and publishes the quality measure specifications, then distributes these specifications and associated terminology through two online resources: the Value Set Authority Center (VSAC), which provides standardized value sets, and the eCQI Resource Center (eCQI RC), which offers implementation guides and educational materials. Providers use these resources to implement the specifications, generate, and submit data measuring the care given. CMS receives the results to support quality improvement efforts nationwide.
CMS National Quality Strategy
The Quality Mission of the CMS National Quality Strategy is to achieve optimal health and well-being for all individuals. The Quality Vision is shaping a resilient, high-value American health care system delivering high-quality, safe, and equitable care for all.
Universal Foundation
The goal of the Universal Foundation is to align quality measures across CMS and advance the vision of the National Quality Strategy. Interoperability is a goal of the National Quality Strategy and several of the initial list of Universal Foundation measures are eCQMs.
Meaningful Measures 2.0
CMS’s Meaningful Measures 2.0 promotes innovation and modernization of all aspects of quality, addressing a wide variety of settings, stakeholders, and measurement requirements.
The eCQM Strategy Project
The eCQM Strategy Project supported the CMS Patients Over Paperwork initiative to evaluate and streamline regulations with a goal to reduce unnecessary burden, to increase efficiencies, and to improve the beneficiary experience. The project provided CMS with an understanding of eCQM implementation and reporting burden and made recommendations for improvement in the use of eCQMs in CMS quality reporting programs. Read the report here.