The Public Comment Period for Draft CMS FHIR® Digital Quality Measures (dQMs) is now open. Comment on Eligible Clinician dQMs, Hospital - Inpatient dQMs, and Hospital - Outpatient dQMs until February 25, 2026.
Top
dQM Subnav
The Centers for Medicare & Medicaid Services (CMS) has set the goal of advancing quality measurement by transitioning all quality measures used in its reporting programs to dQMs.
CMS Digital Quality Measures Defined
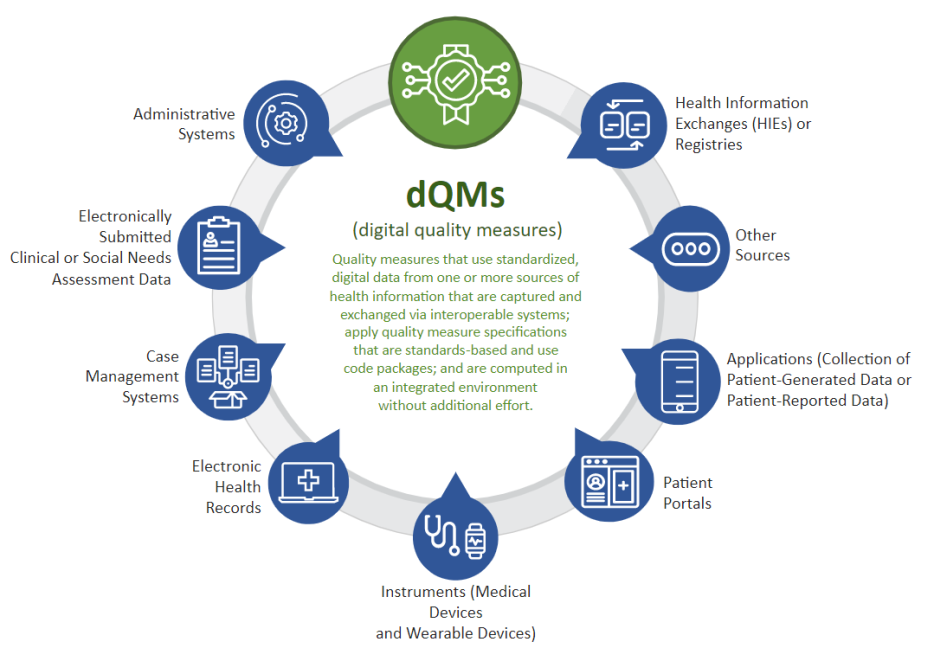
CMS defines dQMs as quality measures that use standardized, digital data from one or more sources of health information that are captured and exchanged via interoperable systems; apply quality measure specifications that are standards-based and use code packages; and are computable in an integrated environment.
The set of solutions for dQMs enables querying the data needed from standards-based application programming interfaces (APIs) (such as Fast Healthcare Interoperability Resources [FHIR®] APIs), calculating the measure score, and generating outputs necessary for quality reporting, that also support quality improvement efforts.
dQMs improve patient care and experiences by ensuring patient and provider access to necessary information, improving the quality of care, the health of populations, and reducing costs due to the rapid-cycle nature of digital health data and dQM calculation.
Why Digital Quality Measures Matter
Data standardization and interoperability support digital quality measurement. In digital quality measurement, the data used are digital, standardized, and a seamless outgrowth of data generated from routine workflows. Data sharing is standards-based to maximize interoperability, minimize burden, and facilitate the development and use of common tooling across use cases. This approach supports data analysis, rapid-cycle feedback, and quality measurement that are aligned for continuous improvement in patient-centered care. As interoperability standards and technology evolve, quality information could become available in near real-time, aiding in rapid improvement to patient care.
Digital Data Sources
Some examples of digital data sources for dQMs are:
- administrative systems
- electronically submitted clinical or social needs assessments
- electronic health records (EHRs)
- laboratory systems
- prescription drug monitoring programs
- instruments (for example, medical devices and wearables)
- patient portals or applications (for example, for collection of patient-generated health data such as home blood pressure monitoring or patient-reported health data)
- health information exchanges (HIEs)
- clinical registries
Transitioning to Digital Quality Measures
Digital Quality Measures (dQMs) represent the next evolution in healthcare quality measurement, building upon the foundation established by electronic Clinical Quality Measures (eCQMs). eCQMs are a subset of dQMs, focused primarily on assessing whether care delivered to patients adheres to evidence-based medicine or clinical guidelines. Traditionally, eCQMs have relied on retrospective data from electronic health records (EHRs) to evaluate clinical performance.
FHIR-based data modeling enhances the scope and capability of quality measurement by enabling more consistent, interoperable, and computable clinical data across systems. This approach allows dQMs to incorporate data from a broader array of sources—including devices, systems, and applications outside of traditional EHRs. The adoption of Health Level 7 (HL7)’s Fast Healthcare Interoperability Resources® (FHIR®) and related standards enables consistent representation and sharing of health data among clinicians, organizations, and patients.
This transition supports real-time quality assessment, reduces manual data entry errors, and enhances scalability across healthcare settings. By harmonizing standards and aligning measure components, tools, and terminology, dQMs facilitate more precise, actionable, and holistic quality improvement efforts. The integration of Clinical Quality Language (CQL) as a standard authoring language further enables dQMs to express clinical logic in a way that is both human-readable and machine-executable.
In summary, while eCQMs remain essential for measurement, dQMs—powered by FHIR-based standards—enable a more dynamic, interoperable, and comprehensive approach to quality measurement, supporting continuous learning and improvement throughout the healthcare ecosystem.