The Public Comment is now open through March 25, 2026 on the Draft 2027 CMS QRDA I Implementation Guide, Schematron, and Sample File for Hospital Quality Reporting
Top
Resources Subnav
Standardized Tools and Resources for Digital Quality Measures (dQMs) & Electronic Clinical Quality Measures (eCQMs)
The use of standardized tools and resources promotes consistency in the development of dQMs and eCQMs. Standardization streamlines implementation and helps reduce the overall burden.
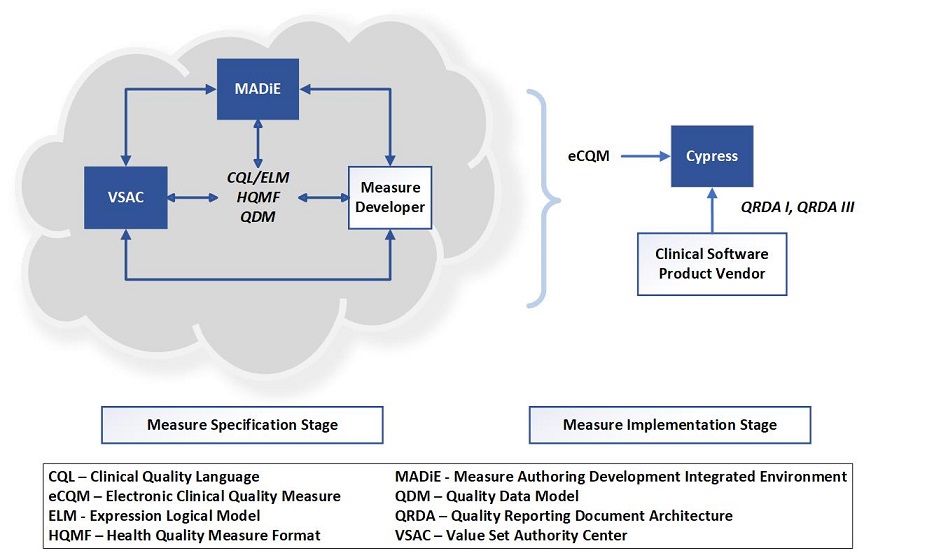
- Measure Authoring Development Integrated Environment (MADiE): a web-based authoring tool required for developing and maintaining dQMs and eCQMs for CMS quality reporting programs. Use of the MADiE tool ensures measure developers are using the established health IT standards and clinical terminology code systems needed for dQM and eCQM implementation. Specifically, the MADiE tool enables measure developers to author in Health Quality Measure Format (HQMF) using the Quality Data Model (QDM) data elements, Clinical Quality Language (CQL), Fast Healthcare Interoperability Resources (FHIR) and other standards to meet future measure authoring requirements.
- Cypress: an open-source testing tool used by health information technology (IT) vendors to certify their electronic health records (EHRs) and health IT modules (CEHRT) for calculating eCQMs. The Cypress application includes the Cypress Validation Utility + Calculation Check (CVU+). The CVU+ facilitates real-world testing, providing health IT vendors the ability to perform QRDA validation testing using their own test patients.
- United States Core Data for Interoperability (USCDI) + Quality Data Element List: a standardized set of health data classes and constituent data elements for nationwide, interoperable health information exchange.
- Value Set Authority Center (VSAC): provides the ability to develop value sets from the Unified Medical Language System terminologies.
The figure below shows connections among eCQM standards and tools used to help develop and test eCQMs.
Find additional eCQI tools on the individual tools and resources tab of each standard on the main menu of the eCQI Resource Center and within the eCQI Tools & Key Resources.
Last Updated: Jan 15, 2026