Resources Subnav
Overview
The QRDA is the data submission standard used for a variety of quality measurement and reporting initiatives. It is based on the Health Level Seven International® (HL7®) Clinical Document Architecture (CDA). QRDA creates a standard method to report quality measure results in a structured, consistent format and can be used to exchange eCQM data between systems.
QRDA further constrains CDA Release 2 for exchange of eCQM data. QRDA was adopted by the Assistant Secretary for Technology Policy/Office of the National Coordinator for Health Information Technology (ASTP) as the standard to support both QRDA I (individual patient) and QRDA III (measured entity’s aggregate) data submission approaches for quality reporting.
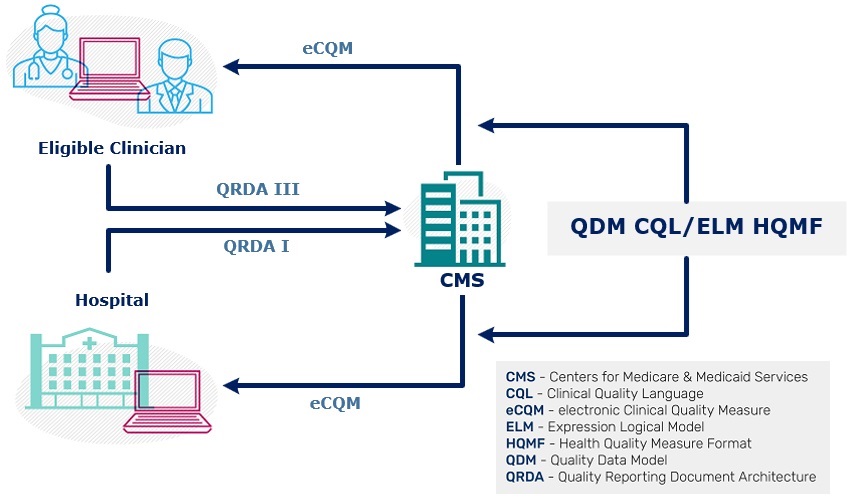
- QRDA I is an individual patient-level report. It contains quality data for one patient for one or more eCQMs. It is implemented by hospitals to submit QRDA I data to CMS.
- QRDA III is an aggregate quality report. A QRDA III report contains quality data for a set of patients for one or more eCQMs, It is implemented by eligible clinicians to submit QRDA III data to CMS.
The HL7 Clinical Quality Information Workgroup maintains and updates both HL7 QRDA I and III standards to ensure alignment with other quality-related standards.
CMS publishes QRDA implementation guides (IGs), schematrons, and sample files annually to provide technical guidance for implementing the HL7 QRDA I and III standards for reporting to CMS quality reporting programs. The CMS IGs further constrain the HL7 QRDA standards to support CMS specific requirements, such as requiring CMS program names. The CMS IGs also provide submission guidance for a specific performance/reporting period. Schematron files contain a list of assertion rules used to validate that the generated QRDA reports conform to the requirements specified in the IGs.
Note: IGs, schematrons, and sample files may be updated after initial publication to address stakeholder or policy requirements. Revisit this page for updated resources prior to use.
Current QRDA Reference and Implementation Guides:
Find QRDA Known Issues in the ONC QRDA Known Issues Project.