
The Quality Data Model (QDM) is a conceptual information model that defines clinical patient data and concepts in a standardized format to enable electronic quality performance measurement. The model is the current structure for electronically representing quality measure concepts for stakeholders involved in electronic quality measure development and reporting. Electronic health record systems can use the QDM to consistently understand the information to access from clinical records to report the data required to meet measure criteria.
CMS established the Quality Data Implementation (QDI) User Group to ensure broad stakeholder collaboration in quality measurement standards development, maintenance, and harmonization with quality measure and Clinical Decision Support (CDS) standards to support the electronic clinical quality improvement (eCQI) landscape. Participants of this open stakeholder group use their expertise to provide feedback and influence electronic data modeling based on
- measurement needs
- real-world feasibility
- harmonization with Health Level Seven International® electronic clinical quality measure (eCQM) and CDS standards.
A broader focus of this group now allows for discussion around emerging quality measurement standards like Fast Healthcare Interoperability Resources® (FHIR®) and Quality Improvement (QI) Core in addition to the QDM data model.
You can review the QDI User Group Charter for more information about the group. To get involved or provide feedback, refer to the Connect tab.
Overview
The QDM provides the language that defines the criteria for clinical quality measurement. The graphic shows how QDM allows for the electronic definition of a clinical concept via its data elements and provides the vocabulary to relate these elements to each other. By relating attributes between data elements and using filtering functions, the QDM provides a method to construct complex clinical representations for eCQMs. The QDM provides the foundation for the conceptualization and specification phases in the eCQM Measure Lifecycle.

Category - Consists of a single clinical concept identified by a value set. A category is the highest level of definition for a QDM element. QDM 5.6 contains 22 categories. Some examples of categories are Medication, Procedure, Condition/Diagnosis/Problem, Communication, and Encounter.
Datatype - The context in which each category describes a part of the clinical care process. Examples of QDM datatypes include “Medication, Active” and “Medication, Administered” as applied to the QDM Medication category.
Attribute - Provides specific details about a QDM data element. QDM data elements have datatype-specific attributes.
- Datatype specific attributes - Datatype-specific attributes provide detail about a QDM data element based on its datatype. For example, “Medication, Dispensed,” “Medication, Order,” and “Medication, Administered” all contain information about dosage, supply, frequency, and route. “Medication, Dispensed” and “Medication Order” include the attribute refills, but “Medication, Administered” does not. Because these attributes pertain to specific datatypes, they are called datatype-specific attributes. Starting with QDM version 5.5 and continuing with subsequent versions, each QDM datatype includes an actor to allow reference to the individual or organization performing the activity. The QDM documentation provides definitions of all QDM attributes including the new actor attributes.
Entities - QDM 5.5 and subsequent versions introduced a new concept called QDM Entities. Entities are not QDM datatypes or attributes. Use entities to represent concepts to specify details about the actor (or performer) of any QDM datatype. An eCQM can use the entities to provide further information required for an individual or organization actor to meet the measure’s criteria. Entities include Patient, Care Partner, Practitioner, and Organization. QDM 5.6 provides examples of how to use these new QDM Entities.
Connections among Standards
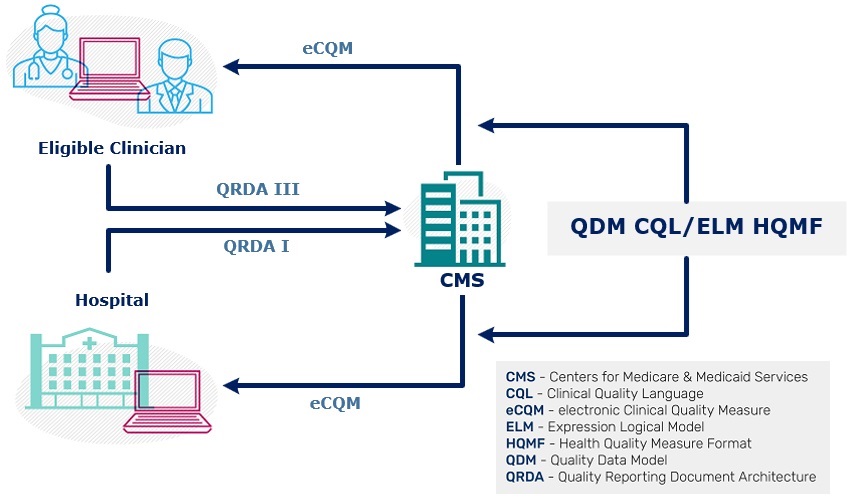
The graphic depicts the flow of quality reporting data between CMS and health care organizations. Health care organizations receive the measure specifications, expressed in Health Quality Measure Format (HQMF) using QDM and Clinical Quality Language (CQL) and then report results to CMS using Quality Reporting Document Architecture (QRDA).
Relationship to future use of FHIR
For those using FHIR resources or are considering transitioning to FHIR for interoperability, the Quality Improvement Core (QI-Core) Implementation Guide provides mapping from QDM datatypes and attributes to QI-Core profiles and elements. The maps may assist implementers in understanding the meaning of QDM datatypes and attributes.
Current Version of the QDM in Use
The most recent version of the QDM is QDM v5.6. It was published January 2021 and lists all changes from QDM v5.5 Guidance Update in section 7.1 and highlights all data model changes throughout the document with red text.
Previous QDM Version Information
For reference, visit the Standards and Tools Versions Chart for more detail on versions used to create and/or support the implementation of the specific reporting/performance period specifications.
Visit the QDM FAQs page to review common questions and answers on QDM.
Find QDM Known Issues on the Clinical Quality Language (CQL) Formatting and Usage Wiki.
Introduction to Quality Data Model (QDM)
- Pioneers in Quality Video Shorts - eCQM Basics Series 1 - January 7, 2021 (Free registration is required to view the videos)
- What is a QDM Data Element Webinar / Transcript
- What is a Datatype - Webinar / Transcript
- What is an Attribute - Webinar / Transcript
- Visit the QDM FAQs page to review common questions and answers on QDM.
QDM v5.6
- Clinical Quality Language (CQL) 1.5 and QDM v5.6 Overview Slides - September 2, 2021
QDM and Quality Improvement Core
- eCQM Transition from Quality Data Model to Quality Improvement Core presentation Slides - August 5, 2021
The Quality Data Implementation (QDI) User Group, formerly the QDM User Group, is a group of volunteer members who use the QDM and Fast Healthcare Interoperability Resources®-related standards for measure development and measure implementation. The QDI User Group meets at 3:00 p.m. ET on the third Wednesday of every month. Meetings last for 90 minutes.
View the QDI User Group Charter and past QDI User Group Meeting Notes for more information about the group.
If you are interested in participating in the QDI User Group, you may download the calendar appointments from the eCQI Resource Center Events page. To join the QDI User Group distribution list, send an email to qdm@icf.com.
Seek Help and Provide Feedback
Post technical questions and comments related to the development and implementation of QDM and questions about the feasibility of capturing information pertinent to the QDI User Group to the ONC Jira QDM Issue Tracker.
