Provide Feedback on draft changes to the 2026 CMS QRDA III IG in the ONC Project Tracking System (Jira) ticket by June 17th.
The Quality Reporting Document Architecture (QRDA) is the data submission standard used for a variety of quality measurement and reporting initiatives. It is based on the Health Level Seven International® (HL7®) Clinical Document Architecture (CDA). QRDA creates a standard method to report quality measure results in a structured, consistent format and can be used to exchange eCQM data between systems.
QRDA further constrains CDA Release 2 for exchange of eCQM data. QRDA was adopted by the Assistant Secretary for Technology Policy/Office of the National Coordinator for Health Information Technology (ASTP) as the standard to support both QRDA I (individual patient) and QRDA III (measured entity’s aggregate) data submission approaches for quality reporting.
The HL7 Clinical Quality Information Workgroup maintains and updates both HL7 QRDA I and III standards to ensure alignment with other quality-related standards.
CMS publishes QRDA implementation guides (IGs), schematrons, and sample files annually to provide technical guidance for implementing the HL7 QRDA I and III standards for reporting to CMS quality reporting programs. The CMS IGs further constrain the HL7 QRDA standards to support CMS specific requirements, such as requiring CMS program names. The CMS IGs also provide submission guidance for a specific performance/reporting period. Schematron files contain a list of assertion rules used to validate that the generated QRDA reports conform to the requirements specified in the IGs.
Note: IGs, schematrons, and sample files may be updated after initial publication to address stakeholder or policy requirements. Revisit this page for updated resources prior to use.
Find QRDA Known Issues in the ONC QRDA Known Issues Project.

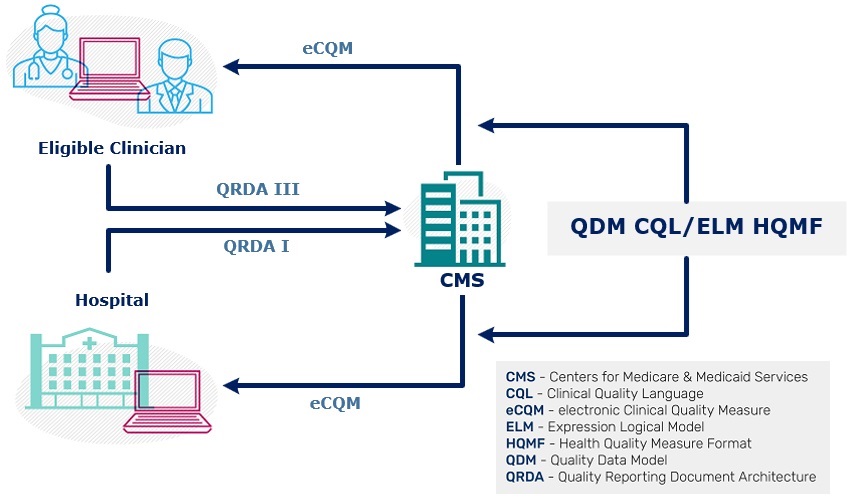
The graphic depicts the flow of quality reporting data between CMS and health care organizations. Health care organizations receive the measure specifications, expressed in HQMF using QDM and CQL and then report results to CMS using QRDA.
The 2026 CMS QRDA I Implementation Guide for Hospital Quality Reporting for 2026 eCQM reporting is based on the HL7 CDA Release 2: QRDA Category I, Release 1, Standard for Trial Use Release 5.3 with errata (published December 2022).
The 2025 CMS QRDA I Implementation Guide for Hospital Quality Reporting for 2025 eCQM reporting is based on the HL7 CDA Release 2: QRDA Category I, Release 1, Standard for Trial Use Release 5.3 with errata (published December 2022).
The 2025 CMS QRDA III IG, Schematron, and Sample Files for Eligible Clinicians for 2025 eCQM reporting is based on the HL7 Implementation Guide for CDA Release 2: QRDA Category III, Release 1 (published September 2021).
The 2024 CMS QRDA I Implementation Guide for Hospital Quality Reporting for 2024 eCQM reporting is based on the HL7 CDA Release 2: QRDA Category I, Release 1, Standard for Trial Use Release 5.3 with errata (published December 2022).
The 2024 CMS QRDA III IG, Schematron, and Sample Files for Eligible Clinicians for 2024 eCQM reporting is based on the HL7 Implementation Guide for CDA Release 2: QRDA Category III, Release 1 (published September 2021).
Please note - Previous versions are for reference only. Do not use these versions for submissions to CMS.
2023 CMS QRDA I
2023 CMS QRDA III
2022 CMS QRDA I
2022 CMS QRDA III
2021 CMS QRDA I
2021 CMS QRDA III
CMS QRDA Pre-Submission Validation Tools - The intent of the Validation Tools Resource is to give users a single point of reference for these tools and assist them in selecting the most appropriate tool to meet their individual needs.
QRDA III Conversion Tool – An open source tool for converting QRDA III files to the Quality Payment Program (QPP) JavaScript Object Notation (JSON) format for data submission to the QPP. Using this tool measure developers can test their ability to create a QRDA III file for successful conversion to the QPP JSON format.
Find QRDA Known Issues in the ONC QRDA Known Issues Project.
Visit the eCQI Tools & Key Resources Library for a complete list of tools and resources used with eCQMs and eCQI.
Participate in an upcoming Health Level Seven International® (HL7®) Clinical Quality Information Workgroup call. This Workgroup creates and maintains HL7 standards supporting quality measurement, evaluation, and reporting.
Post questions and comments related to the development and implementation of QRDA to the ONC Jira QRDA Issue Tracker. You must have an account to enter a question or comment.
Find QRDA Known Issues in the ONC QRDA Known Issues Project.