CQL
Clinical Quality Language – expression language used to communicate specific data to be retrieved and logic needed to evaluate eCQMs. The machine-readable Expression Logical Model (ELM) is a representation of the CQL.
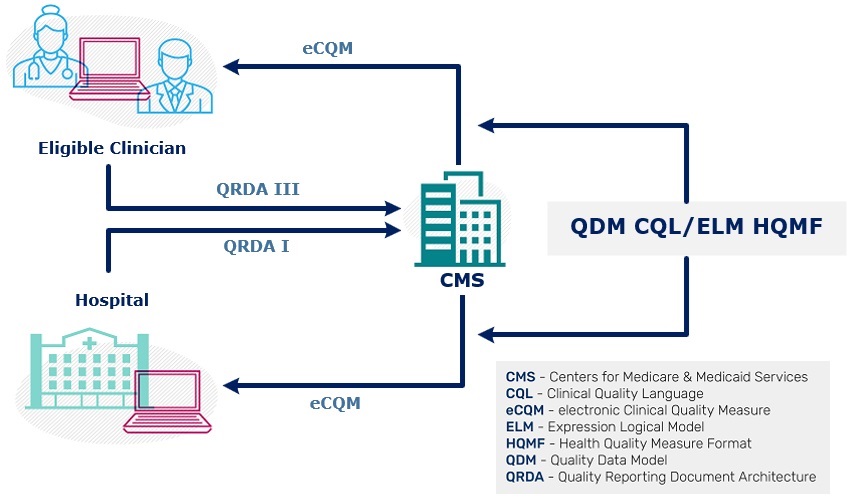
Electronic clinical quality measure (eCQM) standards are critical to data consistency, validity, and interoperability. Standards, in the context of health information technology, refer to agreed-upon methods and terminology for connecting systems such as file formats for electronic documents, messages, and related health care data elements. Standards pertain to data transport, data format and structure, and the meanings of codes and terms. See the Interoperability Standards Platform for more information about eCQI standards and initiatives.