Measure Collaboration (MC) Workspace
Primary tabs
MC Workspace
The MC Workspace brings together a set of interconnected resources, tools, and processes to promote transparency and better interaction across stakeholder communities that develop, implement, and report electronic clinical quality measures (eCQMs).
MC Workspace Components

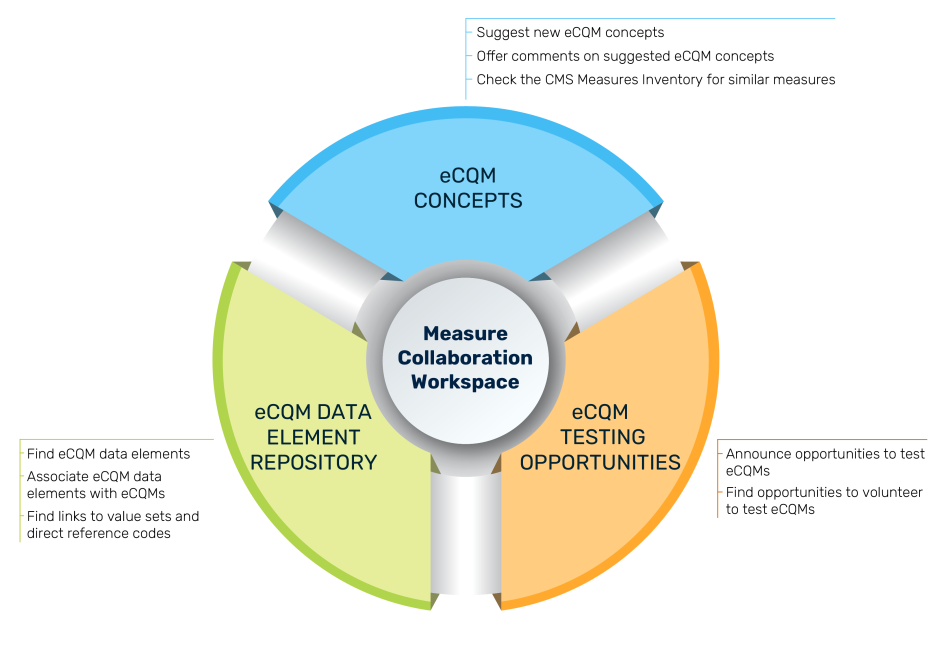
The graphic describes the three modules of the MC Workspace, the purpose of which is to assist clinicians, eCQM developers, implementers, and submitters during the entire eCQM Lifecycle, from initial eCQM concept, through development, implementation, and reporting to CMS. Goals of the MC Workspace are to
- Provide detailed data element definitions to support implementation
- Achieve harmonization across eCQMs, data elements, and value sets
- Improve alignment of eCQM concepts with clinical need and newly published guidelines
- Demonstrate how new eCQMs fill existing quality reporting gaps
- Increase involvement by clinical experts and electronic health record (EHR) vendors during eCQM development
- Provide notification of updates to eCQMs under development
See the MC Workspace User Guide to learn more about how to use the MC Workspace Components.
eCQM Data Element Repository (DERep)
The eCQM Data Element Repository provides all the data elements associated with eCQMs in CMS quality reporting programs, as well as the definitions for each data element. This online searchable repository improves clarity of required data elements for those implementing eCQMs. Visit the eCQM Data Element Repository.
eCQM Concepts
The eCQM Concepts module provides users the ability to comment on eCQM concepts suggested by others, suggest new eCQM concepts, and find links to sources to identify whether similar eCQMs exist. User feedback can help guide an eCQM developer to refine a concept and purpose of a new eCQM to better meet the needs of those interested in quality measurement. Visit the eCQM Concepts module.
eCQM Testing Opportunities
The eCQM Testing Opportunities module provides stakeholders with available opportunities to participate in eCQM testing. Measure developers use testing for a variety of reasons throughout the Measure Lifecycle. Visit the eCQM Testing Opportunity module.
MC Workspace Education
Featured Research Resources
Browse these featured links to identify whether a similar measure concept exists or to get more information about CMS measures:
- CMS Measures Inventory Tool (CMIT)
- Partnership for Quality Measurement (PQM) Submission Tool and Repository (STAR) Measure Database
- Meaningful Measures Initiative
- Pre-rulemaking and Measures Under Consideration (MUC)
- Pre-rulemaking eCQMs for Eligible Hospitals/Critical Access Hospitals
- Pre-rulemaking eCQMs for Eligible Clinicians
Use the MC Workspace to support the eCQM Lifecycle
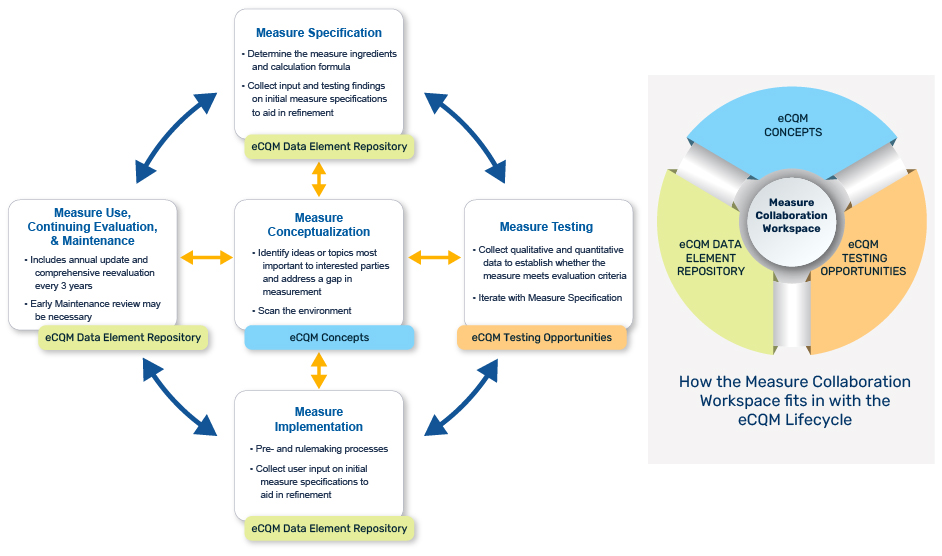
This graphic highlights the key MC Workspace modules for use during various eCQM Lifecycle stages. Learn more about the eCQM Lifecycle.
